Pipeline
Arrien Pharmaceuticals licensed ARN-4079, an IND stage oral JAK3 selective inhibitor (now called OR-101) for dermatology indications, licensed to Ornovi, Inc., in August 2021.
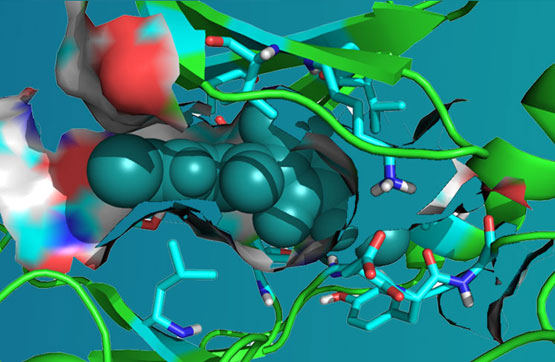
ARN-4079 is a novel, highly selective, irreversible, orally available Janus Kinase 3 (JAK3) inhibitor we have developed, and it targets unique Cys909 residue at the gatekeeper position. ARN-4079 selectively inhibited JAK3 over other JAKs and Cys909 kinome members. A cellular selectivity study demonstrated that ARN-4079 preferentially inhibited JAK3 over JAK1 in JAK/STAT signaling.
ARN-4079 covalently bind to the Cys909 residue in JAK3 was clearly validated and the supportive favorable target profiles, the pharmacokinetic properties and its low toxicity, ARN-4079 exhibit improved efficacy than clinically approved pan-JAK or dual JAK1/JAK3 inhibitors. In our CIA and oxazolone-induced Atopic Dermatitis (AD) mice efficacy studies without any significant adverse effects, ARN-4079 exhibited promising efficacy, safety in RA and AD mice over clinically approved agents.
ARN-4079 is a potent, selective, and durable inhibitor of JAK3 and has the potential for the treatment of inflammatory disorders and autoimmune diseases such; rheumatoid arthritis, atopic dermatitis, alopecia areata, vitiligo and other skin diseases.